| |
1.1*
|
| | | |
| |
2.1*
|
| | | |
| |
2.2*
|
| | | |
| |
3.1*
|
| | | |
| |
3.2*
|
| | | |
| |
3.3*
|
| | | |
| |
6.1*
|
| | | |
| |
6.2*
|
| | | |
| |
6.3*
|
| | | |
| |
6.3.1*
|
| | | |
| |
6.4*
|
| | | |
| |
6.5*
|
| | | |
| |
6.5.1*
|
| | | |
| |
6.6*
|
| | | |
| |
6.7*
|
| | | |
| |
6.8*
|
| | | |
| |
6.9*
|
| | | |
| |
6.10*
|
| | | |
| |
6.11*
|
| | | |
| |
10.1*
|
| | | |
| |
11.1*
|
| | | |
| |
12.1*
|
| | | |
| |
13.1
|
| | |
Chief Executive Officer
(Principal Executive Officer)
| |
Signature
|
| |
Title
|
| |
Date
|
|
| |
/s/ Jason DiBona
Jason DiBona
|
| |
Chief Executive Officer
(Principal Executive Officer) |
| |
November 3, 2021
|
|
| |
/s/ Ryan Tyler
Ryan Tyler
|
| |
Chief Financial Officer
(Principal Financial Officer) |
| |
November 3, 2021
|
|
| |
*
Amin J. Khoury, PhD (Hon)
|
| |
Chairman of the Board
|
| |
November 3, 2021
|
|
| |
*
David Helfet, M.D.
|
| |
Director
|
| |
November 3, 2021
|
|
| |
*
Michael Senft
|
| |
Director
|
| |
November 3, 2021
|
|
| |
*
Thomas P. McCaffrey
|
| |
Director
|
| |
November 3, 2021
|
|
| |
*
Heather Floyd
|
| |
Director
|
| |
November 3, 2021
|
|
Attorney-In-Fact
Exhibit 13.1

Information Deck November 202 1

This presentation includes forward - looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, included in this presentation regarding forward - looking statements. The words “believe”, “anticipate”, “intend”, “expect”, “target”, “goal”, “estimate”, “plan”, “assume”, “may”, “will”, “predict”, “project”, “would”, “could” and similar expressions are intended to identify forward - looking statements, although not all forward - looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward - looking statements, and you should not place undue reliance on our forward - looking statements. We have based these forward - looking statements on our current expectations and projections about future events, nevertheless, actual results or events could differ materially from the plans, intentions and expectations disclosed in, or implied by, the forward - looking statements we make. Factors that could cause such differences, but are not limited to, are our strategy, future operations, regulatory process, future financial position, future revenue, projected costs, prospects, plans, objectives of management and expected market growth. 2 FORWARD LOOKING STATEMENTS

No money or other consideration is being solicited, and if sent in response, will not be accepted . No offer to buy the securities can be accepted and no part of the purchase price can be received until the offering statement filed by AeroClean Technologies (the “Company”) with the Securities and Exchange Commission (the “SEC”) has been qualified by the SEC . Any such offer may be withdrawn or revoked, without obligation or commitment of any kind, at any time before notice of acceptance given after the date of qualification . An indication of interest involves no obligation or commitment of any kind . A copy of the offering circular may be obtained at : https : //www . sec . gov/Archives/edgar/data/ 1872356 / 000110465921131723 /tm 2123085 - 8 _ 1 aa . htm . MARKET AND INDUSTRY DATA Unless otherwise indicated, information contained in this presentation concerning the Company’s industry and the markets in which it operates, including the Company’s general expectations and market position, market opportunity and market size, is based on reports from various sources . Because this information involves a number of assumptions and limitations, you are cautioned not to give undue weight to such information . While the Company has not independently verified market data and industry forecasts provided by any of these or any other third - party sources referred to in this presentation, it believes such sources to be reliable and is not aware of any misstatements in such information . In addition, projections, assumptions and estimates of the Company’s future performance and the future performance of the industry in which it operates are necessarily subject to a high degree of uncertainty and risk due to a variety of factors . These and other factors could cause results to differ materially from those expressed in the estimates made by third parties and by the Company . Each trademark, trade name or service mark of any other company appearing in this presentation is the property of its respective holder . 3

Issuer AeroClean Technologies, Inc. Offering Type Initial Public Offering Securities Offered Shares of Common Stock Price Range $10.00 - $12.00 Shared Offered 2,500,000 shares plus 15%, 45 - day overallotment option Gross Proceeds $27.5 Million at the midpoint of the range Listing/Symbol Nasdaq: AERC Pre - Offering Shares Outstanding 11,363,636 Use of Proceeds Organization build - out & production expansion Joint Book Running Managers: The Benchmark Company, HCFP/Capital Markets, Valuable Capital Limited The Offering 4

About Us AeroClean is a disruptive, interior space air purification technology company with differentiated, medical - grade technology. We are currently initiating full - scale commercialization and FDA clearance of our high - performance interior air sterilization and disinfection products for the eradication of harmful airborne pathogens, including coronavirus (“COVID - 19”), in healthcare and other settings. 5

Our History and Founders Amin J. Khoury Co - Founder/Chairman • B/E Aerospace: Built from a start up to create a $13B enterprise value public company; applied advanced engineering to create industry leader in commercial aerospace cabin interiors. • Synthes: Global leader in orthopedic surgical implants established through organic development in the U.S. and international acquisitions to create global Synthes. Sold to J&J for $21B. David Helfet Co - Founder/Chief Medical Officer • Chairman Emeritus Orthopedic Trauma Service – Hospital for Special Surgery and New York Presbyterian Hospital, Weill Cornell Medicine • One of the world’s leading orthopedic trauma surgeons • Director, Synthes, Inc. Mark Krosney Co - Founder/Chief Scientific Officer • B/E Aerospace, Inc. - VP and General Manager, Business Jet Group • Carnegie Mellon University – MSc, Engineering • Prodigious and creative inventor, holds numerous patents 6 A collaboration between a world - renowned entrepreneur, a trauma surgeon and an inventor who came together to seek a solution to a massive problem of hospital acquired infections and the growing danger of pathogens to our environment .

• Intensive R&D over a 7 - yr period – patented, proven and proprietary technology • Rapid scalability of manufacturing expected to drive incremental revenue growth • Installed base expected to drive recurring consumables and service revenues • Timing is favorable – CDC, EPA and ASHRAE recommending localized, supplemental airborne pathogen eradication • Medical - grade (FDA - regulated) products targeted at $12B niche, limit competition and increase barriers to market entry • Early market acceptance by hospitals, non - hospital healthcare, universities and offices AeroClean Technologies Investment Value p 7 7

Ryan Tyler Chief Financial Officer • B/E Aerospace, Inc.; KLX Inc. • VP – Corporate development and financial reporting for a ~$5B enterprise value public company Rao Tella VP of Operations and Engineering • Nellcor Puritan Bennett - VP of Operations, AeroSystems Business • B/E Aerospace, Inc. – VP and General Manager of the Oxygen Products Business Jason DiBona Chief Executive Officer • 15+ year sales and management veteran at GE Healthcare • EVP of sales, marketing and business development at ePreop, Inc., (healthcare IT start - up with a successful exit) Management Team 8 • First class team with track record of becoming a category leader in competitive environments • Executive team with backgrounds in healthcare, manufacturing, engineering, R&D, and sales at the highest levels of industry - leading organizations yields strategic approach to brand creation, market penetration and cash flow generation

$26+B Total addressable U.S. market $14.6B U.S. non - healthcare opportunity $9.2B: Education • Public schools K - 12 • Private schools K - 12 • Universities and colleges $5.0B: Elevators • Commercial offices • Residential • Hospitals $0.5B: Childcare • Daycare • Pre - schools $11. 8 B U.S. healthcare opportunity $5.1B: Long - term care • Assisted living • Nursing homes • Independent living $4.3B: Non - hospital • Medical offices • Dental offices • Surgery and Outpatient centers $2. 4 B: Hospital • Operating rooms • Waiting rooms • Hospital beds Market Opportunity 9

Early Opportunities by Vertical 10 Hospital & Non - Hospital Healthcare 34% Long - term Care 22% Education 20% Hospitality & Leisure 9% Commercial / Restaurants 15%

Returning to NORMAL life • 85% of all COVID - 19 transmission is airborne 1 person - to - person in the form of aerosolized droplets and in enclosed spaces. • Traditional HVAC systems neither exchange nor purify the air sufficiently to mitigate risk, nor do they eliminate airborne pathogens . Annual Health and Business Impact • $50 Billion – US cost of flu and respiratory infections 2 • 4 Million Pre - mature deaths from Air Pollution 3 • 540,000 Hospital Acquired Infections (HAIs)and 73,000 deaths 4 annually from Airborne Pathogens The P r oblem 11 1 - (NCBI/NIH) 2 - (Journal of Science) 3 - (WHO) 4 - (CDC)

Government Support for enhanced Air Quality Experts push for regulatory overhaul and new workplace standards for AIR QUALITY • Scientific and clinical communities pushing for Indoor Air Quality (IAQ) requirements for all public and commercial spaces. 1906 – Food Safety Act 1971 – Lead Paint Ban 1972 – Clean Water Act • As a result of the pandemic, Germany and other EU countries implementing IAQ standards - requires IAQ monitoring, HVAC upgrades, localized/supplemental room air technology, and new building requirements. • The CDC, EPA and ASHRAE all support the use of portable filtration devices and ultraviolet germicidal irradiation (“UVGI”) air cleaning technology as a proven way to limit the transmission of airborne bacterial and viral infections. • The CDC recommends UVGI as a supplemental air cleaning measure to inactivate SARS - CoV - 2. 12 Crisis often results in reform

Pūrgo Ρ is the SOLUTION Medical - grade device utilizing patented and proprietary SteriDuct ҹ technology to continuously sanitize the air and destroy 99.99% of airborne viruses, bacteria and fungi. Compact & powerful About the size of a carry - on suitcase, yet powerful enough to meet the air quality safety standards outlined by ASHRAE and the CDC . Low maintenance Easy to use with virtually no maintenance needed. Pūrgo only requires a simple filter cartridge replacement every six months and service every 18 months . Active sanitization Continuous cleaning and killing of viruses and superbugs in occupied spaces, yielding four to six air changes per hour. Patented UV - C LED Pūrgo uses SteriDuct ҹ , a proprietary technology that safely maximizes UV irradiation to effectively eliminate 99.99% of harmful airborne pathogens . 13

Pre - filter captures large dust particles, pet hair, and extends the life of the HEPA filter Activated carbon for removal of volatile o r g anic c ompound s * HEPA filter removes > 99% of particles down to 0.1 micron SteriDuct’s UV - C LED technology instantly k ills remaining bacteria and viruses Pathogens don’t stand a chance. Pūrgo uses multiple layers of filtration plus SteriDuct’s patented UV - C LED technology to filter and kill harmful airborne pathogens, preventing cross infection in indoor spaces Ready, set, gone. Pūrgo’s multi - step filtration system 14

15 Deactivates, not kills HVAC/UV Bipolar/Hydroxyl Ionization PECO /PCO Pūrgo TM – No Comparison HEPA Only Pūrgo TM Limited air quality impact Used to clean coils Captures, not kills True HEPA captures UV - C LED Kills Emits ions Chemical reaction Filter loading Particle remobilization Potential ozone/byproduct production Particle breakthroughs/ remobilization Produces sanitized, clean air No harmful byproducts Dual scientifically supported tech. (UVGI and HEPA Filtration) Not supported by CDC/EPA/ASHRAE to combat localized airborne threats Single technology 4 - 6 air changes/hour Plug and play installation Low maintenance Minimal air exchange when used alone

Medical - Grade Protection Supplemental, Localized Airborne Pathogen Control • Meets the air quality safety standards outlined by ASHRAE and the CDC for hospital patient rooms, providing powerful protection for patients, including the immunocompromised. » 10 million immunocompromised people in the U.S. » 60,000 cancer patients hospitalized annually from infections » One patient dies every two hours from this complication • Pūrgo’s advanced pathogen killing performance mitigates situational COVID - 19, HAI and outpatient treatment risk, and puts pathogen control where needed. Pūrgo is operating as a Class II Medical Device in compliance with the FDA’s Enforcement Policy for Medical Air Sterilizers 16

17 • Pūrgo kills 99.99 % of harmful pathogens in minutes • Testing completed in “room sized” chambers at multiple independent laboratories • Pūrgo clears the air in all indoor spaces – small to large, in minutes • Pūrgo use results in significant reduction of harmful particles in occupied spaces Viable Particles vs Time 17 Pūrgo ҹ Testing Results

WƵƌŝĮĞĚŝƌ KƵƚ hŶƚƌĞĂƚĞĚŝƌ/Ŷ Pūrgo Lift Ρ Next Generation Protection P ῡ rgo Lift combines hospital grade filtration and ultraviolet germicidal irradiation (UVGI) to safely deliver unparalleled air quality. Ready, set, gone. Pūrgo Lift’s eradication system 18

Pū r g oLif t ҹ Wall & Ceiling Mount 19 PūrgoLift Ρ protects every person, every ride • Provides visible and continuous air purification in elevators • Designed to fit in any elevator, improve ventilation and reduce viral load • Dramatically reduce the probability of cross infection for masked and unmasked riders

2014 - 2019 2020 2021 2H 2022 • 6 YR development of IP and Patent portfolio • Engineered to reduce HAIs in the operating room • Testing in multiple laboratories to meet FDA medical device criteria • Expected FDA 510k clearance of Pūrgo • Engage best - in - class, 3 rd party regulatory consultants to help build FDA 510k compliance and submission package • Pūrgo complies with U.S. FDA Enforcement Policy for Air Sterilizers • FDA allows AeroClean to market and sell Pūrgo during the Public Health Emergency • Dedicated Quality and Regulatory team and consultants • Comple t ed Pre - submission meeting with FDA to finalize production testing requirements • Finalize submission for 510k Class II Medical Device clearance 20 Regulatory Pathway Pre - Pandemic COVID - 19 Public Health Emergency New Normal

Capital - light Strategy 21 C apital - intensive activities outsourced: Optimizes return on proprietary intellectual property and speed to market FDA certified, contract manufacturing services Numerous engineering and technical resources Regulatory affairs and quality control

Scaling for Demand and Profitability • ACTIVE PIPELINE for 2022 Sales - Approximately $20 million** ($3,250 MSRP) • Recurring Revenues from installed base – o Consumables & Service: Expected to be ~15% of revenues by 2025 • Targeted margins expected to increase with operating leverage to ~50+% 22 0 500 1000 1500 2000 Units Production Actual Projected* • Commercialization - Production and sales began in late July 2021 • Committed production - Through the end of September, production has been sold or committed • Scalable production – • 600 units per month – Anticipated run rate within a few months • 2,000+ units per month - Ramp further with existing tooling during 2022 *Projections are illustrative and assume a linear growth - rate in scale of production to reach projected targets **The Company does not have firm commitments for such amount and cannot guarantee that orders for this amount will be received. The Company’s estimate is based on active discussions by management with prospective customers and management’s estimate of the Company’s contract manufacturer’s ability to increase its production
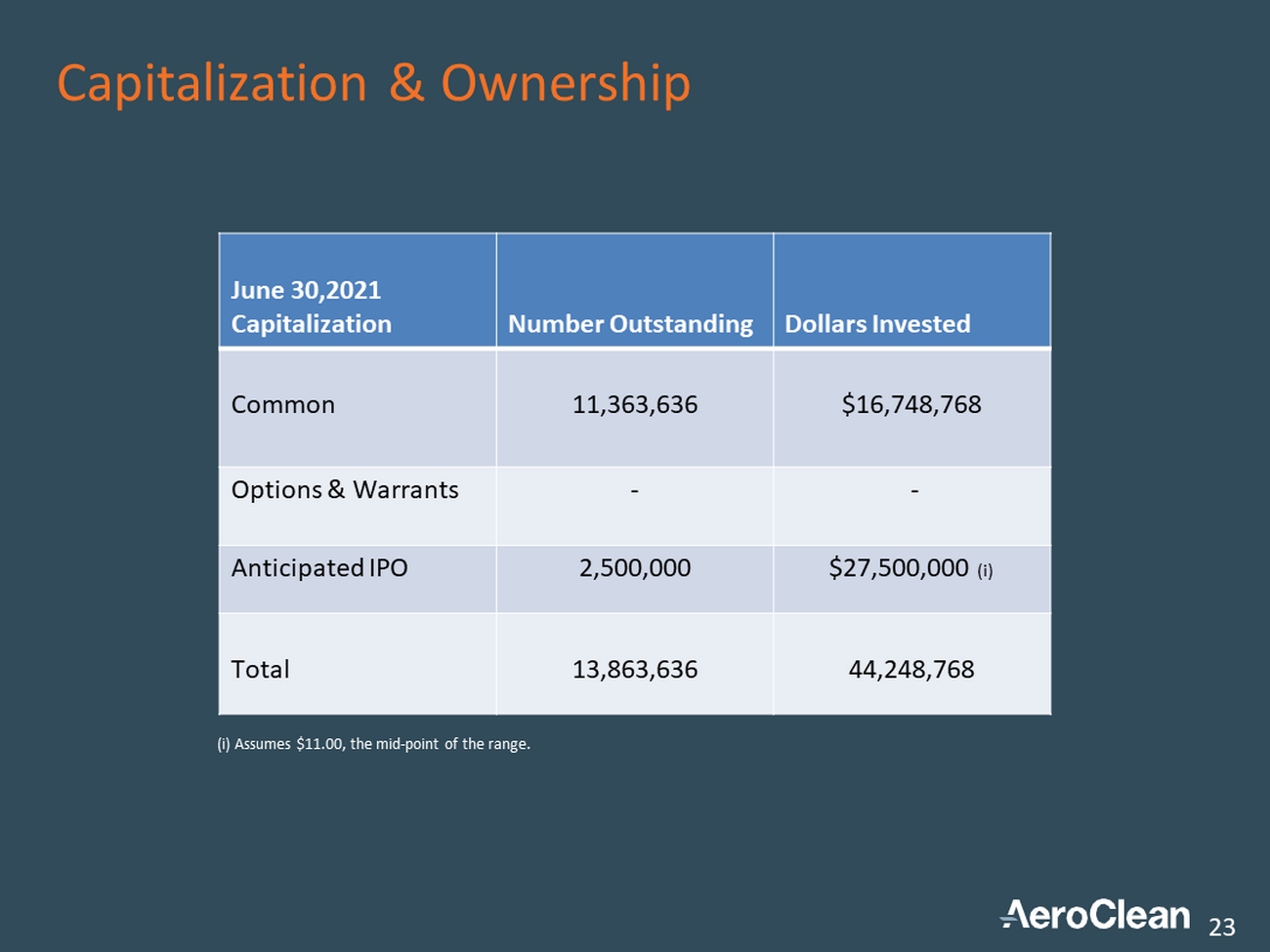
Capitalization & Ownership 23 June 30,2021 Capitalization Number Outstanding Dollars Invested Common 11,363,636 $16,748,768 Options & Warrants - - Anticipated IPO 2,500,000 $27,500,000 (i) Total 13,863,636 44,248,768 (i) Assumes $11.00, the mid - point of the range.

Offering – Fund Operations and Further Innovation 24 Use of Net Proceeds • Continued ramp of Pūrgo production & sales • Development & Launch of Pūrgo Lift • Development & Launch of Service Business • Potential for synergistic, strategic M&A • Cash to balance sheet* 24 Production & Working Capital R&D & Engineering Talent & Infrastructure Cash to balance sheet * *After repayment of bridge loan

With Pūrgo Ρ Life and Work Never Stop 25 With our technology, hospitals, commercial offices, universities and schools, senior living and nursing homes, non - hospital healthcare facilities, and human transport and travel industries are safer, and life keeps going for everyone . MIS SION

Investment Highlights • Significant market demand for innovative, effective air purification solutions such that we can return to normalcy from the COVID - 19 pandemic • Executive team with backgrounds in building and leading international healthcare sales teams and growing large, international public companies both organically and through strategic acquisitions • Platform technology based upon proprietary air purification technology which traces back to efforts to address air quality in commercial aircraft cabins • Recurring revenue model, with ability to scale with limited capital investment • Validation evidenced by early interest and best - in - class testing results • Active pipeline targeting healthcare and non - healthcare verticals 26